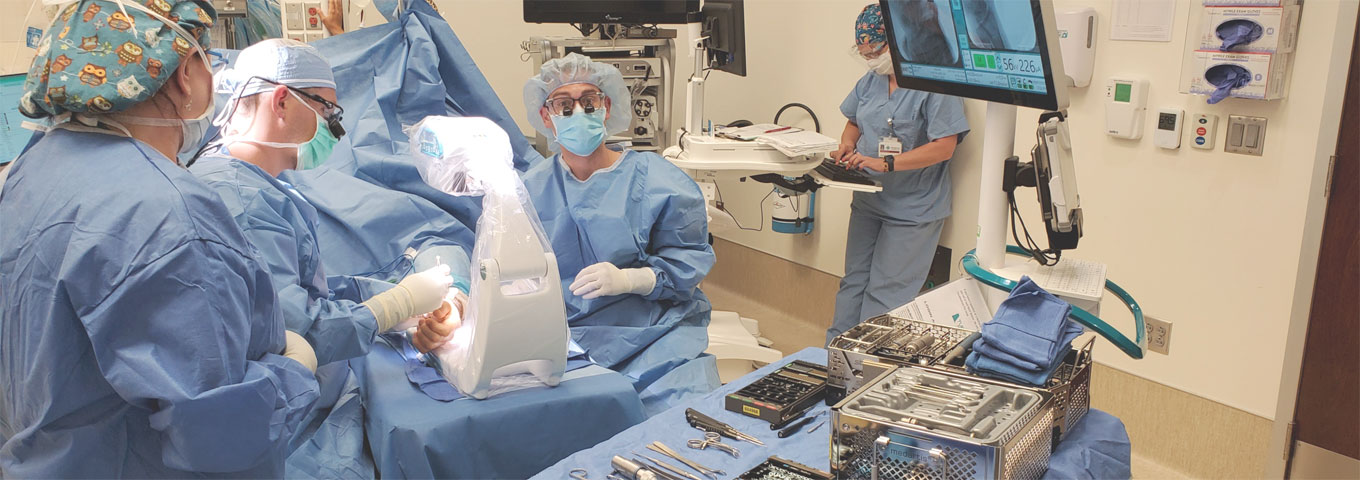
The Challenge
During development of the Turner Imaging Systems’ Smart-C, a partner was needed to accelerate and close out the development process and enable a successful transfer to production for this novel medical imaging device.
The Solution
As an ISO 13485 certified manufacturer and with low overhead costs, Turner MedTech was the perfect choice to partner in the Smart-C development process. As part of this development, the following services were performed:
- Design support – Finalized design details and created detail drawings for dozens of parts.
- Process development – Machined prototype components, created production plan with supporting work instructions, and built systems for V&V testing.
- V&V Testing support – Assumed responsibility for completion of select protocols for product testing.
- Process risk analysis – Completed the risk analysis for Smart-C production processes to assure both product and process safety.
- Process validation – Completed the validation testing to meet IQ/OQ/PQ requirements to successfully transfer the Smart-C into production.
- Production planning – Coordinated with Turner Imaging Systems in production planning, including material sourcing & purchasing, facility setup, personnel, training, etc.
- Production transfer – Providing continuous support in the manufacture and testing of the Smart-C portable imaging system. Design support – Finalized design details and created detail drawings for dozens of parts.
- Process development – Machined prototype components, created production plan with supporting work instructions, and built systems for V&V testing.
- V&V Testing support – Assumed responsibility for completion of select protocols for product testing.
- Process risk analysis – Completed the risk analysis for Smart-C production processes to assure both product and process safety.
- Process validation – Completed the validation testing to meet IQ/OQ/PQ requirements to successfully transfer the Smart-C into production.
- Production planning – Coordinated with Turner Imaging Systems in production planning, including material sourcing & purchasing, facility setup, personnel, training, etc.
- Production transfer – Providing continuous support in the manufacture and testing of the Smart-C portable imaging system.
An Increasingly Smart Solution for Medical Device Manufacturing
This development culminated when the Smart-C received FDA 510k clearance in the fall of 2019, now with hundreds of systems produced and placed both domestically and internationally. From procurement and inventory management, production & product testing, order fulfillment & shipping, and supporting design changes for cost reduction and continuous improvement, the Smart-C Portable Imaging System is a great showcase for Turner MedTech’s capabilities. The partnership employed to complete the Smart-C development is a model for other companies that are looking to accelerate development and successfully launch their new products.
For High Quality Outsourcing, Turner Medtech Prevails